By Nurse Mark
Of all the video presentations Dr. Myatt has made, her presentation on Magnesium Stearate is arguably one of the most popular.
We have heard from countless customers and patients who are grateful to have the hype and B.S. (that’s Bad Science) cleared away for them.
We have heard from supplement manufacturers who have told us that this video presentation is now required watching for their employees as a part of their continuing education because Dr. Myatt has made a complicated subject understandable, even for those without biochemistry degrees.
And we hear from people who probably do have biochemistry degrees, who like to challenge us with all sorts of “have you seen this information that says you are wrong” questions.
Here is a most recent question of the latter sort – this fellow thought that with this information he surely had us:
Dear Dr. Myatt,
I watched your youtube video regarding magnesium stearate. The only thing that wasn’t discussed is the absorption issue. Several studies show that mg stearate lowers the absorption of the supplement drastically.
Here are some studies plus a video which shows an experiment showing the reduced absorption: http://www.ncbi.nlm.nih.gov/pubmed/3735097 , http://www.ncbi.nlm.nih.gov/pubmed/903855 , https://youtu.be/qP8i3_EfP7M
This would make mg stearate not an ideal filling substance or do you see this differently?
Best regards,
Mark , The Netherlands
Dr. Myatt answered Mark with the following information (we are quite familiar with all 3 of the “references” that he provided.):
The two studies you cite are "in vitro," meaning in a test tube.
Absorption will be much different in an actual human ("in vivo") since humans have hydrochloric acid and multiple enzymes to aid digestion and assimilation.
Magnesium stearate is a naturally occurring component of many foods as I discuss in my video. No problems have been seen with decreased assimilation due to ingestion of magnesium stearate containing foods.
If you find any studies that show decreased absorption in humans, I’d be most interested to see them. Until then, I do not have any data or studies or even a theoretical basis to suggest that "mg stearate lowers the absorption of the supplement drastically."
Mark surprised us by emailing back his thanks:
Thanks for the quick response.
I think you are right about the in vitro studies. I haven’t seen in vivo studies yet claiming the same thing. So that’s cleared up for now then!
Thanks again.
Best regards, Mark
A quick review:
We do cover this in the Magnesium Stearate presentation, but it is worth covering again since the third “reference” Mark provided was a “chemistry experiment” offered by someone who should have known better. The doctor presenting this flawed “experiment” has obviously forgotten his anatomy and physiology from his schooling, but he has certainly not forgotten all about marketing. He shamelessly pushes his own brand of supplements at the end of the video, claiming them to be superior because they do not contain mg stearate.
Here is what he forgot to mention in describing his “experiment.”
Distilled vinegar as he used is actually quite a weak acid. Distilled vinegar also contains no other enzymes to aid in the breakdown of mg stearate. He also did not agitate or stir his “experiment” in any way – even though a human stomach is in constant mixing, stirring motion.
pH is a bit of a tough concept – the pH “scale” is logarithmic meaning that for every one point on the scale there is a 10-fold increase or decrease from the next point. This means that the difference between, say, pH 2 and pH 3 is Huge – not minor, and the difference between the pH 1.5 of your stomach acid and the pH 2.4 of vinegar is likewise a Huge difference!
The purest HCL (hydrochloric or ‘sulphuric’ acid) has a pH of 0 – it is as acid as anything can be.
Battery acid, like in your car battery, has a pH of between 0 and 1.
Human gastric acid, as produced by your stomach (if it is healthy) has a pH of around 1 to 1.5.
Distilled vinegar has a pH of around 2.4 and cola has a pH around 2.5.
Pure water has a pH of 7.0 and is considered ‘’neutral.’
Your blood has a pH of 7.4 normally – and that is considered to be very slightly alkaline.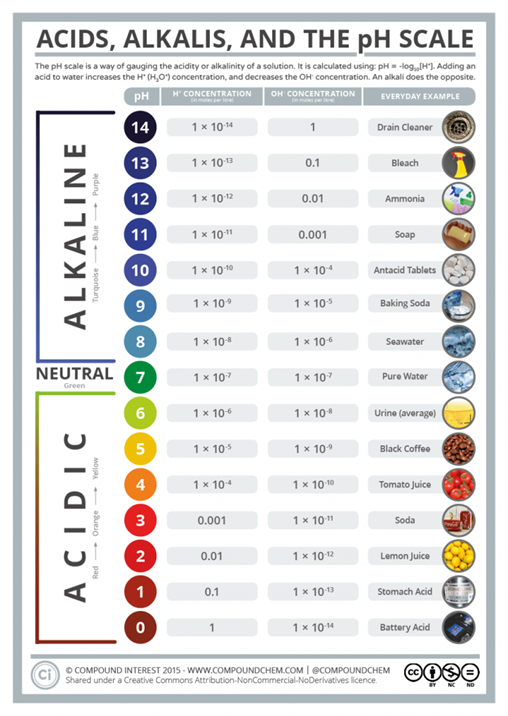
How The B.S. happens:
As you can see, it is easy to get B.S. started. Find a few “experiments” or in vitro (test-tube) studies, mix it all together to prove whatever you want to prove, and let the magic and viral power of the internet do the rest as one person quotes it to another, who quotes it to 10 more, who each then quote it to hundreds… suddenly B. S. has become “truth” since so many people have said it is so!
Why would a doctor use a flawed experiment like this?
It is what is known in marketing as a U.S.P. – a Unique Selling Proposition. It’s the thing that makes this product “special.”
Why is this important?
Let’s face it – when it comes to supplements, there is not much truly “new.” Vitamin C is Vitamin C, no matter how wonderful it’s flavor or packaging.
In order to sell otherwise common vitamins and supplements, marketers need to somehow make their brand more “special” that all the others.
Nano-particles, micronized, hyper-absorbable, energy-infused, and, yes, “free of the evil magnesium stearate.” All these claims are used in an attempt to set one otherwise fairly common product apart from all the others that are just like it.
So, don’t fall for B.S. and grand claims. Be sure to actually verify references and make sure that references are relevant to human beings, not just test tubes.
And please be sure to refresh your memory regarding the B.S. surrounding the Magnesium Stearate “controversy”: